Two large studies presented at the American Heart Association Scientific Sessions 2018 and published in the prestigious New England Journal of Medicine save lives
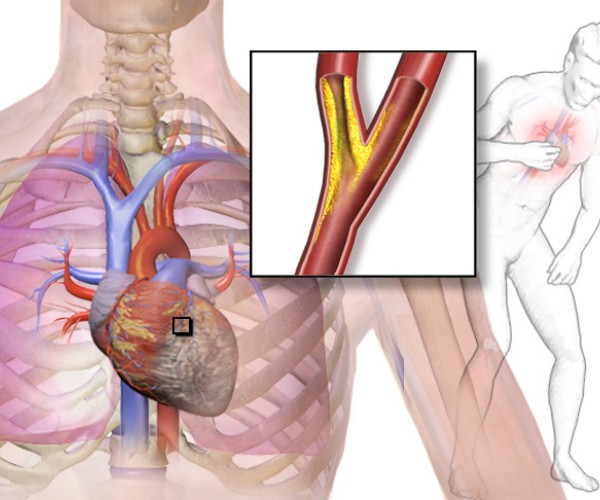
Monza, Nov. 11, 2018 The Omega 3 fatty acid association. with cardiovascular health has been known for forty years now, and over the decades thousands of researches have accumulated a considerable body of data in favor of the hypothesis that they protect the heart and arteries, so much so that Efsa (the European Food Safety Authority) recommends taking at least 250 mg per day of Omega 3 Epa (eicosapentaenoic acid) and Dha (docosahexaenoic acid) to promote good heart function. Now new confirmation of the effectiveness of Omega 3 in protecting cardiovascular health comes from the American Heart Association’s Scientific Sessions 2018 underway in Chicago, Illinois, United States.
Bringing them are the results of two independent large-scale intervention studies. The first, christened REDUCE-IT(Reductionof Cardiovascular Events with Icosapent Ethyl – Intervention Trial), confirms the usefulness of taking adequate doses of Omega 3 to reduce the risk of serious cardiovascular events when living with above-normal triglyceride levels. His results put to rest the doubts raised by recent reviews of the randomized controlled trials on Omega 3, confirming the importance of using an adequate daily dosage of Omega 3 (which in several studies is instead below the amounts needed to observe a significant effect) and of reserving Omega 3 treatment for patients who can really benefit from it because of the cardiovascular risk they are exposed to (in this case, high triglyceride levels). In contrast, the second, VITAL(VITamin D and OmegA-3 TriaL), highlights theeffectiveness of Omega 3 in the primary prevention of myocardial infarction, regardless of the presence of predisposing risk factors.
Expert comments
“The study showed very positive results in terms of both primary endpoints and major secondary endpoints,” said Deepak L. Bhatt, first name of the REDUCE-IT study.
“Those who benefited most from Omega 3 supplements were those who ate little fish“, JoAnn E. Manson, lead author of the VITAL study, explained instead. “We believe that the VITAL study showed no clear reason why those who are already taking fish oil supplements should stop taking them“, Manson added. “And for those who seem to be able to benefit-particularly, in terms of reducing heart attacks with Omega 3, for those who eat little fish-we think it makes sense to talk to your doctor about the possibility of taking these supplements“.
“The results are in line with indications that are about to be published as guidelines by the International Society for the Study of Fatty Acids and Lipids (ISSFAL), the scientific society that deals with Omega 3 internationally” explains Clemens von Schacky, a cardiologist at Ludwig Maximilian University in Munich, Germany, and a world expert on the relationship between Omega 3 and cardiovascular disease. According to von Schacky key parameter to be measured in this type of study is the so-called Omega 3 Index*, an indicator of the amount of Epa and Dha out of the total fatty acids in red blood cell membranes that should be measured before and during trials. “Otherwise ,it’s not clear what happened,” explains von Schacky “In the VITAL study, omega-3 was measured in plasma rather than red blood cells, and the dosage of omega-3 supplementation was not high enough to distinguish between the intervention group and placebo, as also explicitly stated by the authors. In REDUCE-IT, however, the dose of Epa was such that it increased blood levels of Epa by a factor of almost 6-clearly enough to differentiate between intervention and control groups. Of course, measuring the true Omega 3 Index would have provided a clearer picture of the situation“. And it is for this reason, the expert explains, that the REDUCE-IT study had sharper clinically relevant effects on both primary and secondary endpoints.
REDUCE-IT – the study
REDUCE-IT is a controlled clinical trial (i.e., in which the effect of the drug was compared with that of a placebo) randomized (i.e., in which participants were randomly assigned to either the group that took the drug or the group that took the placebo) double-blinded (i.e., in which neither the investigators nor the participants knew who would take the drug and who would take the placebo). Participants were characterized by high triglyceride levels (between 150 and 499 mg/dl), LDL cholesterol levels (the one considered hazardous to cardiovascular health) kept under control (between 41 and 100 mg/dl) by statin therapy, and a previous cardiovascular event or diagnosis of diabetes associated with at least one other cardiovascular risk factor. The first outcome considered was the incidence of death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke, coronary revascularization, or unstable angina.
The study, presented in Chicago last Saturday, November 10, and published simultaneously in the prestigious New England Journal of Medicine, involved 8,179 patients (mean age 64 years, 29% women) recruited from 473 centers in 11 different countries; mean triglyceride and cholesterol levels were 216 and 75 mg/dl, respectively, and 71% of participants were taking treatment for cardiovascular risk reduction. Each patient took either 4 grams of Omega 3 Epa or a placebo daily, and their health status was monitored for an average of 4.5 years. It was found that taking high doses of Omega 3 significantly reduced the risk of cardiovascular events in patients at risk due to high triglyceride levels.
As anticipated last September by Amarin Corporation plc, the manufacturer of the omega-3 drug tested in the study, the overall reduction in deaths from cardiovascular causes, nonfatal myocardial infarctions and nonfatal strokes, coronary revascularization or unstable angina requiring hospitalization was found to be 25 percent. The current event in Chicago was an opportunity to present more detailed results:
- During the first year of treatment, treatment with Omega 3 a reduced triglyceride levels by an average of 39 mg/dl, compared with an increase in the control group of an average of 4.5 mg/dl;
- Omega 3s were found to be effective regardless of triglyceride levels at the beginning of the study;
- With Omega 3, the overall risk of heart attack, stroke or death from cardiovascular causes decreases by 26 percent;
- Omega 3s reduce the risk of nonfatal heart attack or stroke and all-cause mortality by 23%;
- Omega 3 intake reduces the risk of fatal or nonfatal myocardial infarction by 31 percent, the risk of fatal or nonfatal stroke by 28 percent, the risk of urgent or emergent revascularization by 35 percent, the risk of unstable angina or hospitalization by 32 percent, the risk of dying from cardiovascular causes by 20 percent, and total mortality by 13 percent;
- Adverse effects were similar in the two groups. Hemorrhages (detected in 2.7 percent of Omega 3-treated patients and 2.1 percent of those taking placebo) were never fatal, and taking Omega 3 did not increase the incidence of hemorrhagic strokes. Other side effects noted included diarrhea and anemia (more frequent in the control group) and constipation, peripheral edema, and atrial fibrillation (more frequent with Omega 3 intake); hospitalizations for atrial fibrillation or flutter were more frequent among Omega 3-treated patients, but according to Bhatt this finding would be worrisome if the risk of stroke was increased, whereas instead it was found to be reduced by as much as 28 percent.
VITAL – the study
VITAL is also a double-blind randomized controlled clinical trial presented in Chicago last November 10 and simultaneously published in the New England Journal of Medicine. In his case, the 25,871 participants (men and women aged at least 50 and 55 years, respectively) had no significant cardiovascular risk and no history of prior cardiovascular disease. Thus, in this case, the effectiveness of Omega 3 was tested in terms of primary prevention, that is, in healthy individuals.
Omega 3 treatment included daily intake of 1g fish oil containing 840 mg Epa (460 mg) + Dha (380 mg). The average duration of treatment was 5.3 years. The primaryend point was the incidence of major cardiovascular events (myocardial infarction, stroke, and death from cardiovascular causes), while secondary end points included the incidence of the individual cardiovascular events considered. This resulted in a 28% reduction in the risk of myocardial infarction, a 50% reduction in the risk of fatal heart attacks, and a 17% reduction in the risk of coronary artery disease. Among participants who ate little fish, the reduction in heart attacks alone was as much as 40%, while the more general reduction in cardiovascular events was 19%.