A randomized trial examining 75 patients with type 2 diabetes showed the efficacy and safety of an innovative minimally invasive ablative procedure designed to promote regrowth of the duodenal mucosa.
The coordinator of the work was Arun Sanyal, professor of gastroenterology at Virginia Commonwealth University School of Medicine in the United States, who presented the findings at the annual meeting of the American Association for the Study of Liver Diseases (AASLD), held in Boston Nov. 13-17.
Sanal reported improvement in glycemic and liver function-related values and a reduction in body weight, detected six months after performing this endoscopic technique.
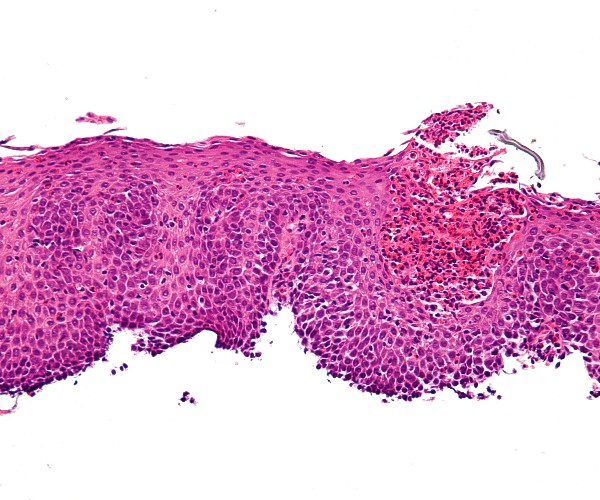
The prospective, double-blinded study took place in nine hospital facilities in the European Union and two in Brazil.Thirty-nine of the participants underwent this procedure, called the endoscopic duodenal mucosal “resurfacing” (DMR) technique, while the other 36 received a sham procedure, similar to the placebo that is administered in this type of study when evaluating drugs.
Before the surgery, the patients had an average glycated hemoglobin (HbA1c) value of 8.3 and body mass index averaged 31.1 kg/m2: in short, they were diabetic and obese subjects. After 24 weeks there was a general improvement, but markedly greater in subjects actually treated with duodenal mucosal ablation, in whom a positive trend was also observed in values related to nonalcoholic hepatic steatosis, a condition that promotes cirrhosis and liver failure.
In addition to efficacy, the procedure was characterized by overall safety: there were no serious adverse events. “Duodenal mucosal hyperplasia,” Sanyal said, “is a potential therapeutic target for metabolic diseases related to insulin resistance.
Source: LO2 presentation at the 2019 Liver Meeting in Boston.